Patient Preference
Data for Natural Thyroid Extract (NTE) Therapy*
Recent research demonstrates a category of patients who report improved symptom relief and preference for an NTE such as ADTHYZA®.1
*The studies evaluated NTE products. ADTHYZA is an NTE product, but was not evaluated in these studies.
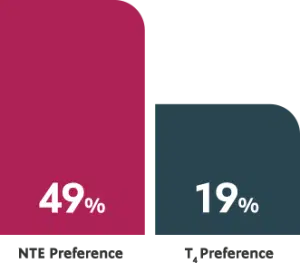
ACTOR PORTRAYALIn a randomized, double-blind, crossover study, patients 18 to 65 years of age (n=70) were randomized to either T4 or NTE and then crossed over for the same duration. At the end of the study, nearly half of patients preferred the NTE.1
With an NTE, patients reported improved:1*
- mood
- Sleep
- energy
- overall cognitive performance (concentration, memory, and decision-making capability)
- weight loss
*As measured by the general health questionnaire (GHQ-12) and thyroid symptom questionnaire (TSQ).
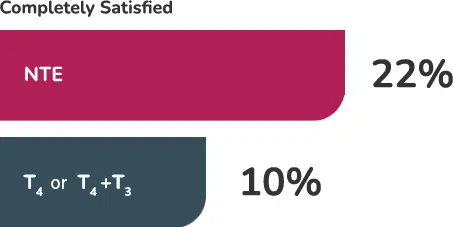
A recent survey of 12,146 respondents with hypothyroidism found that patients being treated with NTE reported being completely satisfied with their treatment more than 2:1 compared to patients taking T
When asked to rate their overall treatment satisfaction, with 1 as “Completely Unsatisfied” and 10 as “Completely Satisfied,” the median scores also favored NTE.2
Additionally, those being treated with NTE were less likely to report sections problems with fatigue/energy levels, mood, weight management, or memory.2

In another randomized, double-blind crossover study, hypothyroidism patients were assigned to 1 of 3 treatment arms: T4, T4 + T3, and NTE. Outcomes were similar across groups. Those patients who were most symptomatic on T4 preferred and responded positively to therapy with T4 + T3 and NTE.3
†Hypothyroidism survey organized by the American Thyroid Association (ATA). This survey was available online from January 28, 2017, to March 30, 2017. Questions identified demographic and treatment characteristics of individuals being treated for hypothyroidism, as well as satisfaction with their therapy. Initial analysis was conducted on sample of respondents taking T4, T4 + T3, or NTE, also known as desiccated thyroid extract (DTE).
Connect on ADTHYZA
Sign up to learn how ADTHYZA may benefit your patients.
T4, levothyroxine; T3, liothyronine.
References
- 1. Hoang TD, Olsen CH, Mai VQ, Clyde PW, Shakir MKM. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98(5):1982-1990. doi:10.1210/jc.2012-4107
- 2. Peterson SJ, Cappola AR, Castro MR, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid. 2018;28(6):707-721. doi:10.1089/thy.2017.0681
- 3. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021;106(11):e4400-e4413. doi:10.1210/clinem/dgab478
Please note that Adthyza® has not been reviewed by the FDA for safety or efficacy.
IMPORTANT SAFETY INFORMATION
WARNING
DRUGS WITH THYROID HORMONE ACTIVITY, ALONE OR TOGETHER WITH OTHER THERAPEUTIC AGENTS, HAVE BEEN USED FOR THE TREATMENT OF OBESITY. IN EUTHYROID PATIENTS, DOSES WITHIN THE RANGE OF DAILY HORMONAL REQUIREMENTS ARE INEFFECTIVE FOR WEIGHT REDUCTION. LARGER DOSES MAY PRODUCE SERIOUS OR EVEN LIFE-THREATENING MANIFESTATIONS OF TOXICITY, PARTICULARLY WHEN GIVEN IN ASSOCIATION WITH SYMPATHOMIMETIC AMINES SUCH AS THOSE USED FOR THEIR ANORECTIC EFFECTS.
INDICATIONS:
ADTHYZA® (thyroid tablets, USP) is a prescription medicine indicated as replacement or supplemental therapy in patients with hypothyroidism of any etiology, except transient hypothyroidism during the recovery phase of subacute thyroiditis.
ADTHYZA® is also indicated as a pituitary TSH suppressant in the treatment or prevention of various types of euthyroid goiters, including thyroid nodules, subacute or chronic lymphocytic thyroiditis (Hashimoto’s), multinodular goiter, and in the management of thyroid cancer.
CONTRAINDICATIONS:
ADTHYZA® is contraindicated in patients with uncorrected adrenal cortical insufficiency, untreated thyrotoxicosis, and apparent hypersensitivity to any component of the product.
The use of thyroid hormones in the therapy of obesity, alone or combined with other drugs, is unjustified and has been shown to be ineffective. Neither is their use justified for the treatment of male or female infertility unless this condition is accompanied by hypothyroidism.
WARNINGS AND PRECAUTIONS:
Thyroid hormones should be used with great caution in circumstances where the integrity of the cardiovascular system is suspected. In the elderly and in patients with cardiovascular disease, ADTHYZA should be used with greater caution. In these patients, therapy should be initiated with low doses of ADTHYZA. When, in such patients, a euthyroid state can only be reached at the expense of an aggravation of the cardiovascular disease, thyroid hormone dosage should be reduced.
Thyroid hormone therapy in patients with concomitant diabetes mellitus or diabetes insipidus, or adrenal cortical insufficiency aggravates the intensity of their symptoms, and appropriate adjustments of the various therapeutic measures directed at these concomitant endocrine diseases are required. The therapy of myxedema coma requires simultaneous administration of glucocorticoids.
Hypothyroidism decreases, and hyperthyroidism increases the sensitivity to oral anticoagulants. Prothrombin time should be closely monitored in thyroid-treated patients on oral anticoagulants, and the dosage of the latter agents should be adjusted on the basis of frequent prothrombin time determinations. In infants, excessive doses of thyroid hormone preparations may produce craniosynostosis.
Carcinogenesis/Mutagenesis: A reportedly apparent association between prolonged thyroid therapy and breast cancer has not been confirmed, and patients on thyroid for established indications should not discontinue therapy. No confirmatory long-term studies in animals have been performed to evaluate carcinogenic potential, mutagenicity, or impairment of fertility in either males or females.
Pregnancy and Lactation: Thyroid replacement therapy for hypothyroid women should not be discontinued during pregnancy, and hypothyroidism diagnosed during pregnancy should be promptly treated. Minimal amounts of thyroid hormones are excreted in human milk. However, caution should be exercised when the thyroid is administered to a nursing woman. Routine determinations of serum T4 and/or TSH are strongly advised in neonates in view of the deleterious effects of thyroid deficiency on growth and development.
ADVERSE REACTIONS:
Adverse reactions other than those indicative of hyperthyroidism because of therapeutic overdosage, either initially or during the maintenance period, are rare.
Many drugs and some laboratory tests may alter the therapeutic response to ADTHYZA®. In addition, thyroid hormones and thyroid status have varied effects on the pharmacokinetics and actions of other drugs. Patients on oral anticoagulants, insulin, and oral hypoglycemics should be monitored closely during the initiation of thyroid replacement therapy.
DRUG INTERACTIONS:
- Oral Anticoagulants: Concomitant use of thyroid hormones with oral anticoagulants alters the sensitivity of oral anticoagulants.
- Insulin or Oral Hypoglycemics: Initiating thyroid replacement therapy may cause increases in insulin or oral hypoglycemic requirements. Patients receiving insulin or oral hypoglycemics should be closely watched during initiation of thyroid replacement therapy.
- Cholestyramine or Colestipol—Cholestyramine or colestipol binds both levothyroxine (T4) and liothyronine (T3) in the intestine, thus impairing the absorption of these thyroid hormones. Four to five hours should elapse between the administration of cholestyramine or colestipol and thyroid hormones.
- Estrogen, Oral Contraceptives—Estrogens tend to increase serum thyroxine-binding globulin (TBg). In a patient with a nonfunctioning thyroid gland who is receiving thyroid replacement therapy, free levothyroxine (T4) may be decreased when estrogens are started, thus increasing thyroid requirements. Patients without a functioning thyroid gland who are on thyroid replacement therapy may need to increase their thyroid dose if estrogen or estrogen-containing oral contraceptives are given.
For further information, please see the accompanying complete Prescribing Information for ADTHYZA.
To report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449 or the FDA at
www.fda.gov/medwatch or call 1-800-FDA-1088.